- Visibility 17 Views
- Downloads 1 Downloads
- DOI 10.18231/j.ijmr.2020.040
-
CrossMark
- Citation
Detection of bioflim production among Staphylococcus.aureus by Congo red method and tube method
- Author Details:
-
Sharlee R *
-
Sumangala B
Introduction
Staphylococcus.aureus is a commensal bacteria on the human skin and mucosa and also a prominent human pathogen that can cause healthcare associated infections and community associated skin and soft tissue infections.[1] Staphylococcus has an ability of acquiring drug resistance and bioflim production in indwelling medical devices making them an important pathogen.[1]
Staphylococcal bioflim can develop on various structures such as prosthetic joints, prosthetic heart valves, catheters, contact lenses, cardiac pacemakers and cerebrospinal fluid shunts.[2] 80% of Nosocomial infections are due to bioflim production. S.aureus is one of the frequently found organisms in bioflim associated infections.[3], [4]
Bioflim appear to be the wise move for bacteria to survive to any kind of environmental stress. The response of bacteria needs to be fast enough to survive those stresses. Bioflim productions by Staphylococcus.aureus begins with adhesion of bacteria to inert /biotic surface with help of adhesion factor microbial surface components recognizing adhesive matrix molecules (MSCRAMMS).[5], [6]
Bacteria forms monolayer and maturation of cell starts when bacteria aggregate and produce slime layer named as Matrix. Matrix contains exopolysaccharides, protein and extracellular DNA.[6], [7] Cell proliferation takes place from monolayer to micro colony and micro colony to bioflim by Quorum sensing system.[8], [9] Quorum sensing system is a cell to cell communication system to coordinate population density dependent changes. Quorum sensing system of S.aureus is autoinducing peptides (AIP) and Agr (Accessory gene regular) induced by an extracellular ligand. Dispersion is the final step of bioflim production. It acts as an important step in expansion of bioflim and also causing systemic dissemination.[8], [9]
Factors that enhances bioflim production in S.aureus are high level glucose, NACL (Sodium chloride), NO (Nitric oxide), MG2+ (Magnesium ion) and in human body, the lack of nutrients (e.g- iron, carbon source) or oxygen.[10], [11], [12], [13]
Host response towards bioflim production: a) S. aureus bioflim secretes specific toxins called leukocidin AB (LukAB) and alpha‐toxin (Hla). These toxins facilitate bioflim production by inhibiting macrophage phagocytosis and induce cytotoxicity, promoting macrophage dysfunction.[14] b) Myeloid‐derived suppressor cells (MDSCs) inhibit T lymphocyte proliferation and prevent macrophage/monocyte pro-inflammatory activity facilitating bioflim persistence.[15] c) Early Th1 and Th17 inflammatory responses are increased and Th2, Treg responses are decreased.[16] Down regulation of Th2 and Treg responses favor the development of S. aureus biofilm infection.[17] Staphylococcus which produces biofilm are more prone to cause disease like endocarditis, urinary tract infections, osteomyelitis, skin and soft tissues infections.[18], [19] Present study was carried out with an interest to isolate the Staphylococcus.aureus from clinical specimens, detect biofilm production and check for the contribution of Methicillin resistancesin biofilm production.
Aims and Objectives
To study bioflim production among Staphylococcus.aureus isolates.
To know the percentage of biofilm production among MRSA.
To compare biofilm detection by Tube and Congored method.
Materials and Methods
Study design
Prospective study.
Study period
6 Months, October 2018-March 2019.
Sample size
150.
Methods of data collection
A total of 150 Staphylococcus.aureus isolates were collected from various clinical samples like urine, pus, sputum, blood and other body fluid received in Microbiology laboratory, Mandya institute of medical science, Mandya.
First, the isolates were identified as Staphylococcus on the basis of colony morphology on Nutrient agar, Blood Agar, Gram’s stain and biochemical tests. The yellow coloured, moist, round, glistening opaque colonies with beta hemolysis on blood agar, Gram positive cocci exhibiting positive test result with respective controls to catalase, coagulase (Slide and tube), nitrate reduction, methyl red, voges proskauer, alkaline phosphatase, urease and fermentative to lactose, mannitol, maltose, mannose, sucrose and trehalose were confirmed as S.aureus.Obtained isolates of S.aureus were screened for Methicillin resistance by inoculating onto mannitol salt agar and performing antibiotic susceptibility testing using Cefoxitin disc by Kirby-bauer disk diffusion method.[20]
A total of 150 isolates were detected for bioflim production by Congored method and Tube method.
Congored method: it is a qualitative assay for detecting of bioflim. Congored medium was prepared using 37g/L of brain heart infusion agar(BHI), 36g of sucrose and 0.8g of congo red.[21] loop fullof colonies from agar plate were inoculated and incubated at 37°C for 24 hours, colour change in colonies were recorded. Bioflim producing isolates showed Black colonies with dry crystalline consistency and non bioflim producers were pink in colour.[21]
Tube method: a loop full of colonies from agar plate were inoculated into Trypticase soy broth supplemented with 1% glucose and incubated for 24 hours at 37°C. Tubes were decanted and washed with distilled water and dried. Dried tubes were stained with 0.1% crystal violet. Excess stain was removed and washed with deionized water, tubes were dried in an inverted position and observed for biofilm formation. Bioflim formation were considered as visible flim lined the wall and bottom of testube. Negative result was taken as ring formation at the liquid interface. Bioflim formation were determined as weak, moderate and strong.[22]
Results
Congored method
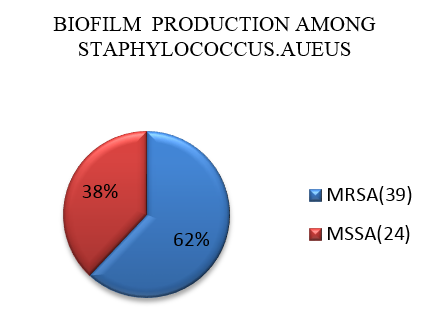
A total of 150 isolates were tested for biofilm production by congored method. Out of 150 isolates, 63 isolates showed black colonies with dry crystalline consistency indicating biofilm production. Out of 63 isolates 39 (62%) isolates were MRSA and 24(38%) isolates were MSSA as shown in ([Figure 1]).
Tube method
A total of 150 isolates were tested by tube method, 84 isolates showed bioflim formation. Out of 84 isolates, 9 isolates were strong biofilm producers, 26 isolates were moderate, and 59 were weak biofilm producers.
Biofilm producers among MSSA were 48.2% and MRSA were 81.53%. In our study we observed that MRSA isolates were significant biofilm producers when compare to MSSA isolates.
|
Total no. of isolates (150) |
Bioflim formation tube method |
|||
|
Strong (%) |
Moderate (%) |
Weak (%) |
Negative (%) |
|
|
MSSA (85) |
3(3.4%) |
15(17.6%) |
23(27%) |
44(51.7%) |
|
MRSA(65) |
6 (9.2%) |
11(17.1%) |
36 (55.3%) |
12(18.4%) |
Out of 85 isolates, 51.70% of isolates were non biofilm producer, 27% were weak biofilm producer, 17.60% were moderate producer and 3.40% were strong biofilm producer.
Out of 65 isolates, 18.40% of isolates were non biofilm producer, 55.30% were weak biofilm producer, 17.10% were moderate producer and 9.20% were strong biofilm producer.
Discussion
In the present study we included 150 Staph.aureus isolates. Out of 150 isolates, 85(56.6%) were MSSA and 65(43.4%) were MRSA. Out of 85 MSSA isolates, 41(48.23%) isolates showed biofilm production by tube method and 24(28.23%) isolates by Congored method. Out of 65 MRSA isolates, 53(81.53%) isolates showed biofilm production by tube method and 39(60%) by Congored method. Our study revealed detection of biofilm by tube method is better than Congored method.
In comparison to our study we found similar type of screening methods used to identify biofilm production. In the study conducted by Malgorzata Piechota et al., out of 130 isolates, 57(43.8%) were MSSA and 73(56.2%) were MRSA. Biofilm producers were about 99.2%. Out of 57 MSSA, 36.8% were strong, 45.6% were moderate and 17.6% were weak biofilm producers. Among 73 MRSA, 39.7% were strong, 47.9% were moderate and 11% were weak biofilm producer.[23]
In the study conducted by Afreenish Hassan et al., showed the comparision of biofilm production by tube method and congored method with respective result. Screening tube method showed 19% strong, 30% moderate and 51% weak biofilm producers, whereas Congored method showed 3.6% strong, 6.4% moderate and 90% weak.[24]
In the study conducted by Maria-Guadalupe Avila-Novoa et al observed among 84 isolates of Staph.aureus, 90.4% were weak and 7.1% were strong biofilm producer by tube method and 75% were biofilm producers by Congored method.[25] Muhammad Sohail et al observed 50% were weak, 27% were moderate and 23% were strong producers.[26]
Conclusion
Biofilms exhibit resistance to antimicrobial agent. Biofilm production among lifesaving devices are untreatable, recurrent and failure of medical devices. Staphylococcus is the major pathogen causing biofilm, so study on Staphylococcus is important to overcome chronic and recurrent infection. In the present study, based on our observation we found tube method as best screening method in comparison to Congored method.
Source of Funding
None.
Conflict of Interest
None.
References
- F D Lowy. Staphylococcus.aureus infections. N Engl J Med 1998. [Google Scholar]
- H Mccarthy, J K Rudkin, N S Black, L Gallagher, E O Neill, J P O Gara. Methicillin resistance and the biofilm phenotype in Staphylococcus aureus. Front Cell Infect Microbiol 2015. [Google Scholar]
- N Hoiby, T Bjarnsholt, M Givskov, S Molin, O Ciofu. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 2010. [Google Scholar]
- U Römling, C Balsalobre. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med 2012. [Google Scholar]
- M Otto. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med 2013. [Google Scholar]
- J M Patti, B L Allen, M J Mcgavin, M Hook. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol 1994. [Google Scholar]
- J Josse, F Velard, S C Gangloff. Staphylococcus aureus vs. Osteoblast: Relationship and Consequences in Osteomyelitis. Front Cell Infect Microbiol 2015. [Google Scholar]
- B R Boles, A R Horswill. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog 2008. [Google Scholar]
- R P Novick. Autoinduction and signal transduction in the regulation of Staphylococcal virulence. Mol Microbiol 2003. [Google Scholar]
- Y Lim, M Jana, T T Luong, C Y Lee. Control of glucose- and NaCl-induced biofilm formation by rbf in Staphylococcus aureus. J Bacteriol 2004. [Google Scholar]
- C D L F Nunez, F Reffuveille, K E F Smith, R Hancock. Effect of nitroxides on swarming motility and biofilm formation, multicellular behaviors in Pseudomonas aeruginosa. Antimicrob Agents Chemother 2013. [Google Scholar]
- J P Farre, J B Förstner, K Engelmann, S Hecker, M. The sigmaB regulon in Staphylococcus aureus and its regulation. Int J Med Microbiol IJMM 2006. [Google Scholar]
- S Rachid, K Ohlsen, U Wallner, J Hacker, M Hecker, W Ziebuhr. Alternative transcription factor sigmaB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J Bacteriol 2000. [Google Scholar]
- T D Scherr, M L Hanke, O Huang, D B A James, A R Horswill, K W Bayles. Staphylococcus aureus Biofilms Induce Macrophage Dysfunction Through Leukocidin AB and Alpha-Toxin. MBio 2015. [Google Scholar]
- C E Heim, D Vidlak, T D Scherr, J A Kozel, M Holzapfel, D E Muirhead. Myeloidderived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. J Immunol Baltim Md 1950. [Google Scholar]
- R Prabhakara, J M Harro, J G Leid, M Harris, M E Shirtliff. Murine immune response to a chronic Staphylococcus aureus biofilm infection. Infect Immun 2011. [Google Scholar]
- R Prabhakara, J M Harro, J G Leid, A D Keegan, M L Prior, M E Shirtliff. Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infect Immun 2011. [Google Scholar]
- D Lebeaux, A Chauhan, O Rendueles, C Beloin. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens 2013. [Google Scholar]
- A E Paharik, A R Horswill. The Staphylococcal Bioflm: Adhesins, Regulation, and Host Response. Microbiol Spectr 2016. [Google Scholar]
- . . Methods for dilution. Antimicrobial susceptibility tests for bacteria that grow aerobically 2018. [Google Scholar]
- D J Freeman, F R Falkiner, C Keane. New method for detecting slime production by Coagulase negative Staphylococci. J Clin Pathol 1989. [Google Scholar]
- G D Christensen, W A Simpson, J J Younger, L M Baddour, F F Barrett, D M Melton. Adherence of coagulase-negative Staphylococci to plastic tissue culture plates: A quantitative model for the adherence of Staphylococci to medical devices. J Clin Microbiol 1985. [Google Scholar]
- M Piechota, B Kot, A F Maciejewska, A Grużewska, A W Kosek. Biofilm Formation by Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus Strains from Hospitalized Patients in Poland. Biomed Res Int 2018. [Google Scholar] [Crossref]
- A Hassan, J Usman, F Kaleem, M Omair, AKhalid, M Iqbal. Evaluation of different detection methods of biofilm formation in the clinical isolates. Braz J Infect Dis 2011. [Google Scholar]
- M G A Novoa, M I Moreno, O A S Velazquez, J P G Gomez, P J G Medina, M G Lomel. Biofilm Formation by Staphylococcusaureus Isolated from Food Contact Surfaces in the Dairy Industry of Jalisco. J Food Qual 2018. [Google Scholar] [Crossref]
- M Sohail, Z Latif. Molecular analysis, biofilm formation, and susceptibility of methicillin-resistant Staphylococcus aureus strains causing community- and health care-associated infections in central venous catheters. Rev Soc Bras Med Trop 2018. [Google Scholar]
